 Finished Medical Products
Finished Medical Products
Insulin Pen Needles – Single-Use Pen Needles
- ISO 11608-2 aligned
- For subcutaneous insulin injection
- Compatible with many insulin pens
- Multiple gauge/length options
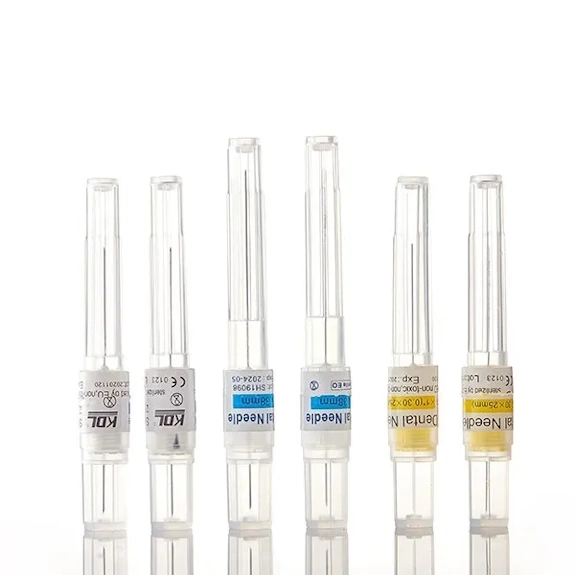
Insulin Pen Needles (Single-Use, Double-Ended)
KDL insulin pen needles are engineered for subcutaneous insulin delivery using compatible insulin pens, supporting comfortable injections and dependable performance in daily diabetes care. The product structure typically consists of a sealing strip, needle holder, needle tube, small sheath, and large sheath, allowing secure installation on the pen and safe protection before and after use.
Key advantages for diabetes care procurement
- Standards-aligned design: the pen needle meets the requirements of ISO 11608-2 for needle-based injection systems.
- Biocompatibility and compatibility considerations: manufacturer indicates ISO 10993 biological evaluation and compatibility testing with insulin drug solution.
- Universal fit approach: designed to be commonly compatible with insulin injection pens on the market (confirm pen models in your tender specification).
- Thin-wall concept can improve flow characteristics, while protective components help reduce accidental touch contamination during handling.
Typical use cases
These pen needles are widely deployed in hospitals, diabetic clinics, pharmacies, and home-care programs (under professional guidance). They are particularly suitable where patients require a consistent injection routine and where medical providers need standardized, single-use consumables across multiple pen brands.
Available configurations (confirm at ordering)
Pen needles are commonly supplied in multiple gauge and length combinations to match patient needs and clinical protocols. For example, safety pen needle variants may be supplied with needle lengths such as 4 mm, 5 mm, 6 mm, and 8 mm across gauges 29G–32G. Confirm the exact configuration needed for your project (gauge, length, and packaging) and we will quote the appropriate item.
Supply and support
Alrowad Pico supports B2B sourcing with bulk quantities, stable production lots, and documentation packages typically requested by procurement teams. Request a quotation to match your target market requirements, desired packaging (e.g., 100 pcs/box), and any labeling or private brand needs.
Important: Single-use medical device. Follow local diabetes care guidelines and dispose of sharps safely.


Technical Specifications
| SKU | KDL-PEN-NEEDLE |
|---|---|
| Intended use | Subcutaneous insulin injection with insulin pen |
| Standards | ISO 11608-2 (needle-based injection systems) |
| Biocompatibility note | ISO 10993 biological evaluation (manufacturer-stated) |
| Structure | Sealing strip; needle holder; needle tube; small sheath; large sheath |
| Material | PE; PP; SUS304 stainless steel cannula; Silicone oil (manufacturer-stated) |
| Configuration options | Multiple gauges & lengths (confirm at ordering) |
| Sterilization | EO (manufacturer-stated for KDL pen needle lines) |
| Shelf life | 5 years (manufacturer-stated on KDL pen needle page) |
| Category | Finished Medical Products |
| Tags | Needles |
Ready to Place Your Order?
Get a customized quote for your business needs. Our team is ready to assist you with bulk orders, technical specifications, and delivery options.









Reviews
There are no reviews yet.